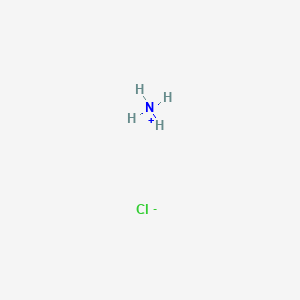
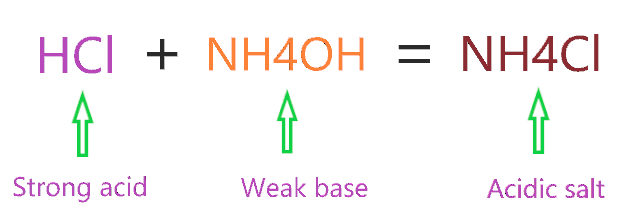
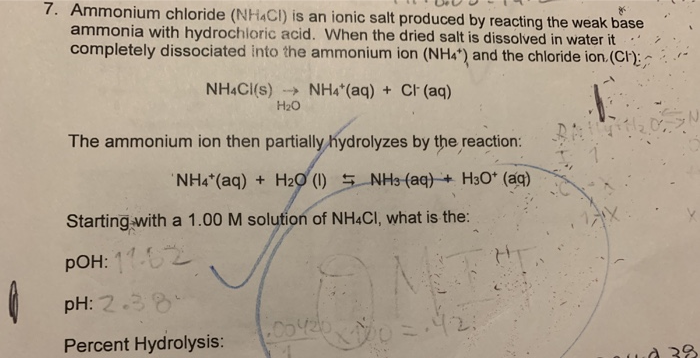Identity the acid and base for ammonium chloride salt. state the nature of this salt and mention its - Brainly.in

Write the chemical formula of ammonium chloride. Explain why an aueous solution of ammonium chloirde - YouTube
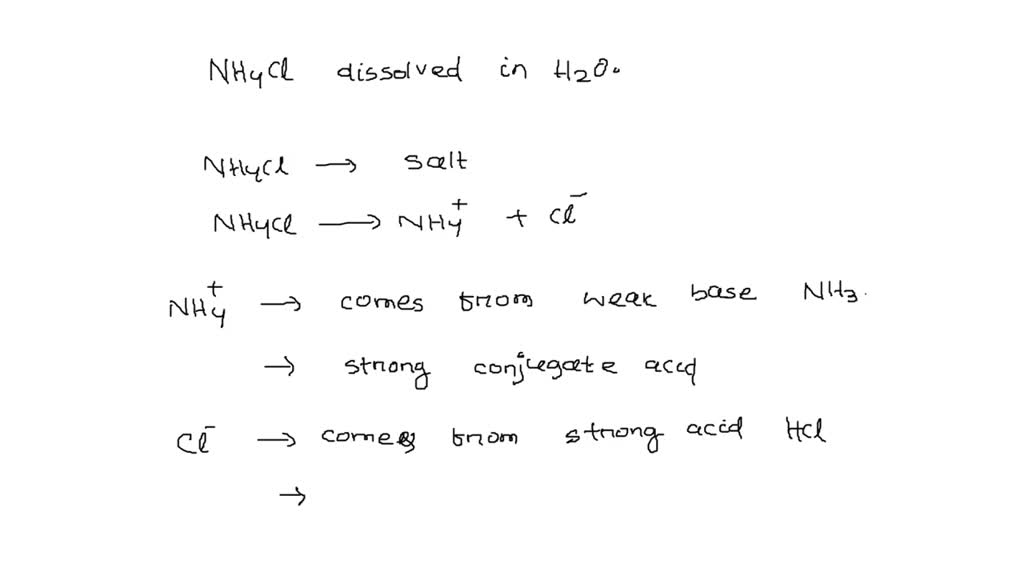
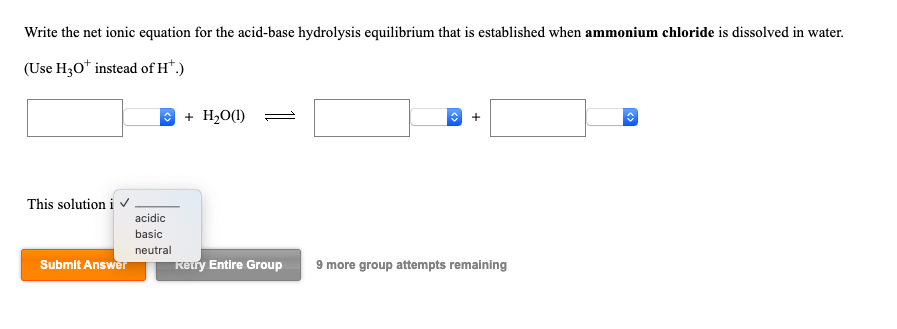
SOLVED: Write the net ionic equation for the acid-base hydrolysis equilibrium that is established when ammonium chloride is dissolved in water. (Use H3O+ instead of H+.) + H2o(l) = + is the

pH calculation of a buffer solution made from a weak base and its conjugate acid (salt form) - YouTube
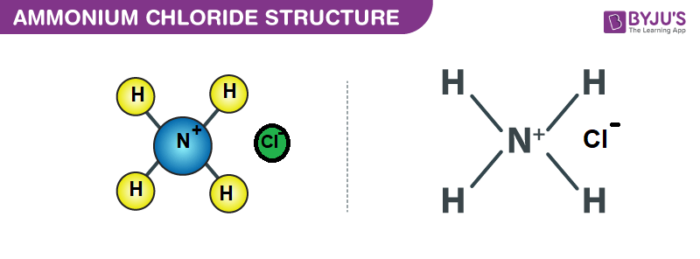
Ammonium Chloride (NH4Cl) - Structure, Properties, Preparation, Uses, Health Risk & FAQs of Ammonium Chloride.
CHEM 1332 (A.M. Guloy) CHEMICAL EQUILIBRIA--ACID/BASE Acid/base problems may fall into 4 categories: strong acid/base, weak acid

SOLVED:A buffer contains significant amounts of ammonia and ammonium chloride. Write equations showing how this buffer neutralizes added acid and added base.