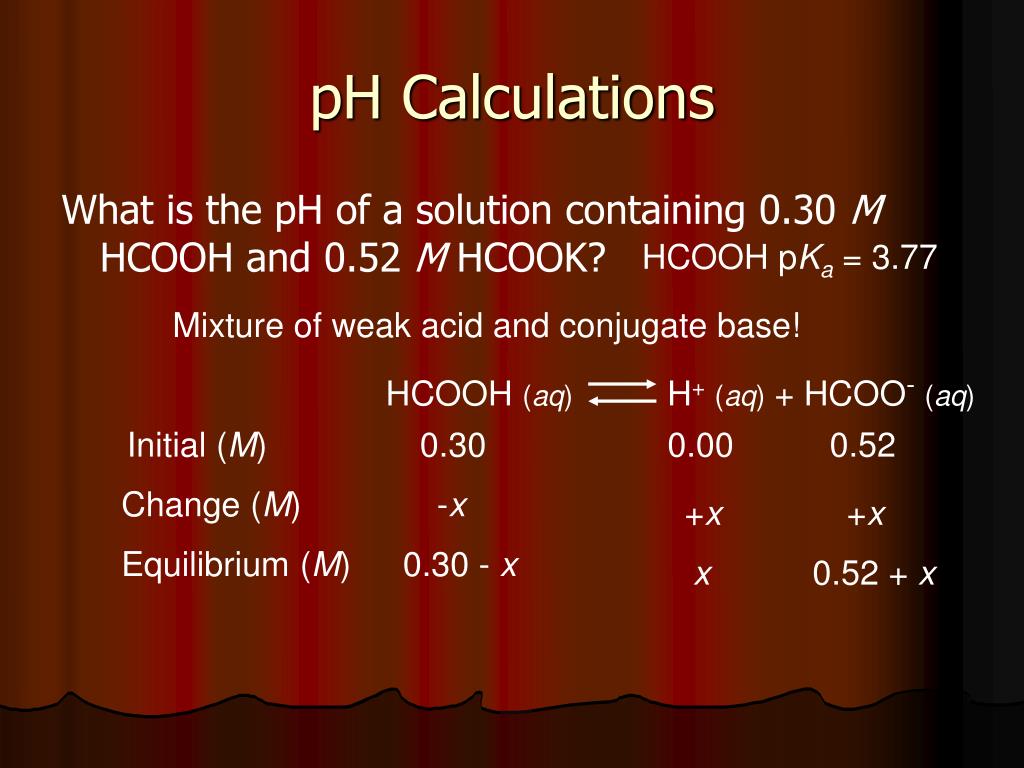
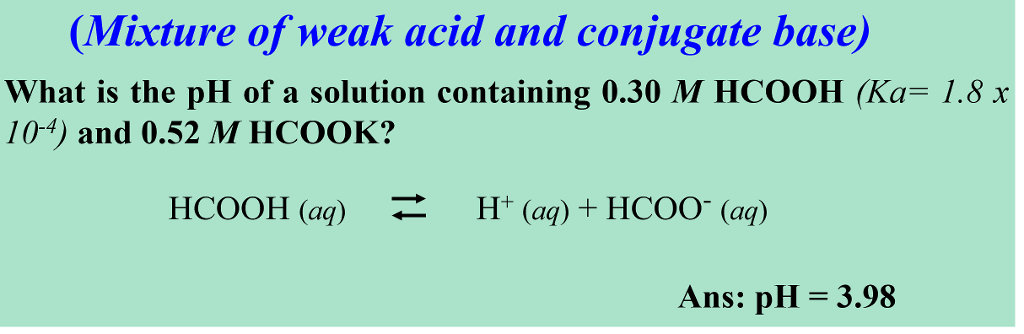
![pH = - log [H + ] or pH = - log [H 3 O + ] Example: If [H + ] = 1 X pH = - log 1 X pH = - (- 10) pH = 10 What would be the pH of a. - ppt download pH = - log [H + ] or pH = - log [H 3 O + ] Example: If [H + ] = 1 X pH = - log 1 X pH = - (- 10) pH = 10 What would be the pH of a. - ppt download](https://images.slideplayer.com/26/8472866/slides/slide_40.jpg)
pH = - log [H + ] or pH = - log [H 3 O + ] Example: If [H + ] = 1 X pH = - log 1 X pH = - (- 10) pH = 10 What would be the pH of a. - ppt download

Acid-Base Equilibria Chapter HF( aq ) + H 2 O( l ) H 3 O + ( aq ) + F - ( aq ) Addition of NaF will shift the equilibrium to the _______because. - ppt download

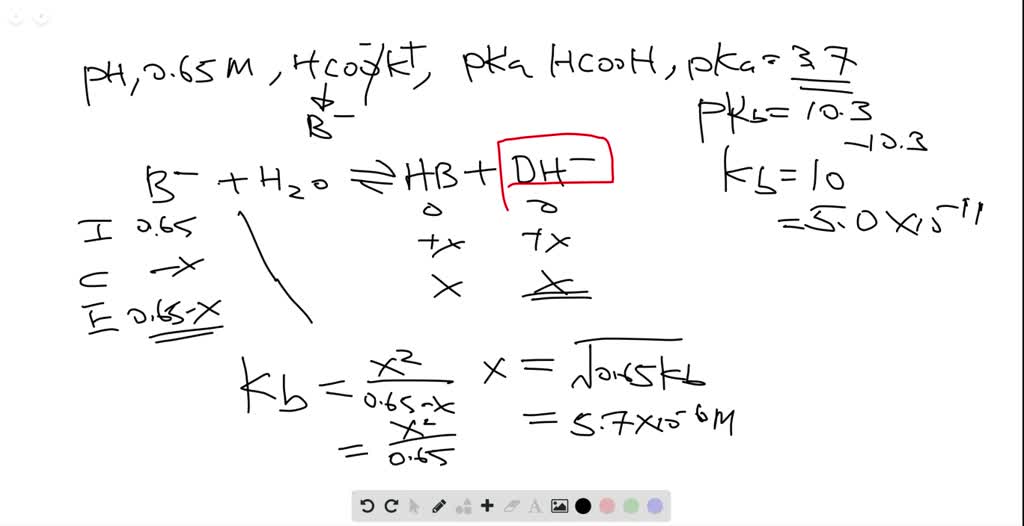
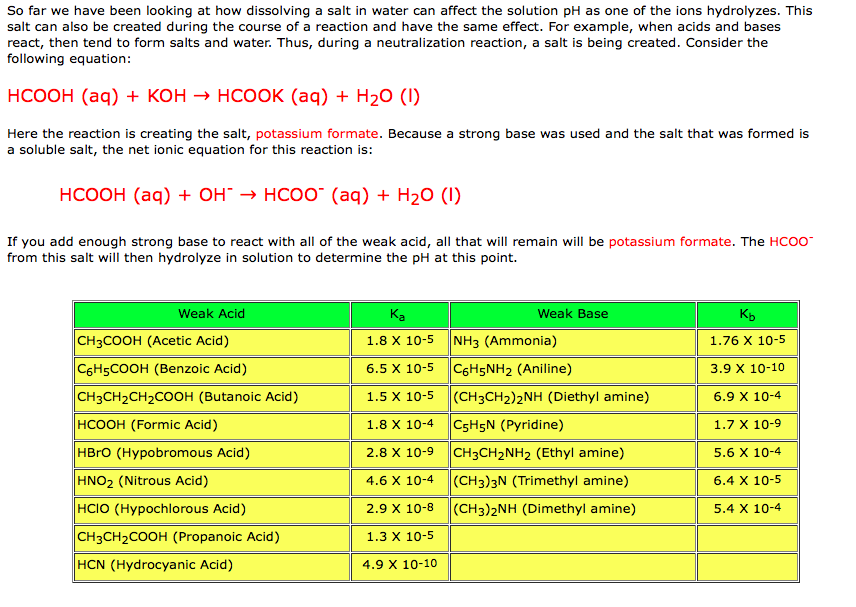
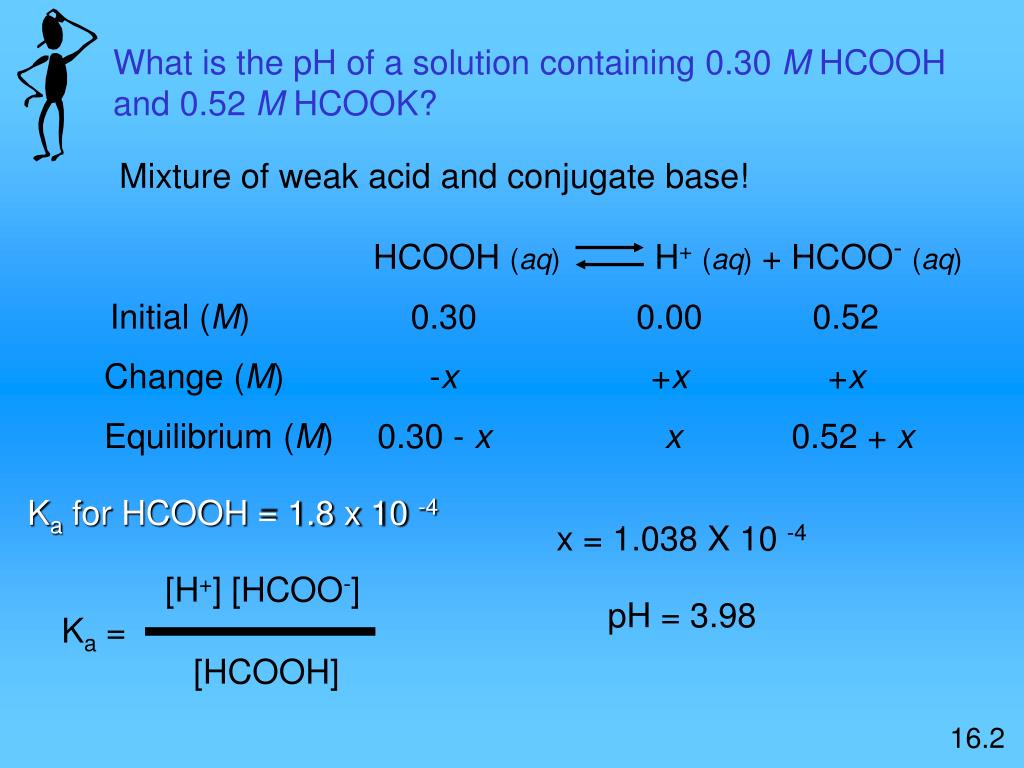
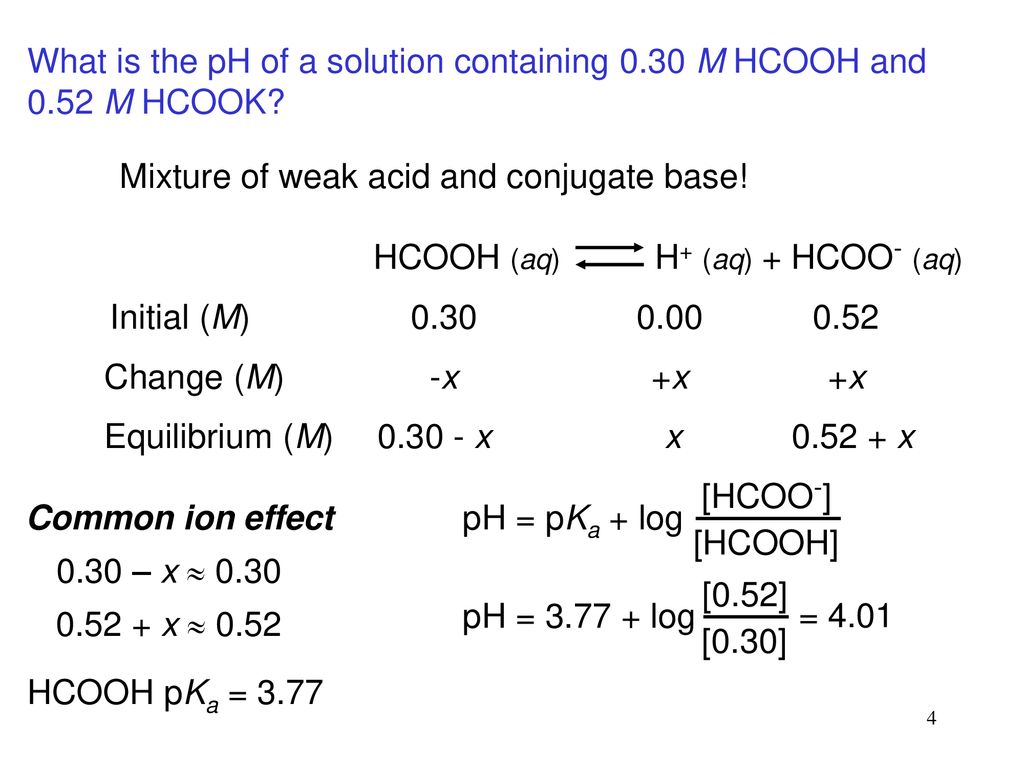
In acid buffer solution (pH = 4.4), the ratio of concentrations of acid to salt is 2 : 1. The value of dissociation constant of weak acid may be:
In situ observation of [Mn-OOCH] by NMR. Reaction conditions: HCOOH (5... | Download Scientific Diagram
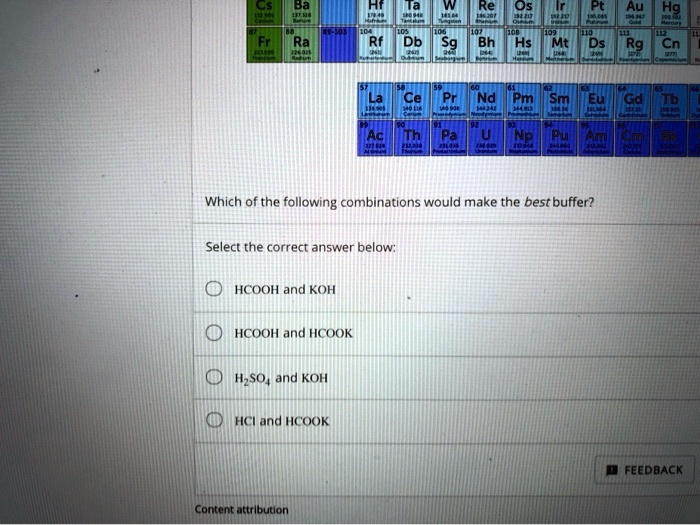
SOLVED: Os 6H Db Sg Hs Mt Ds Rg Cn Cel PN Which of the folllowing combinations would make the best buffer? Select the correct answer below HCOOH and KOH HCOOH and

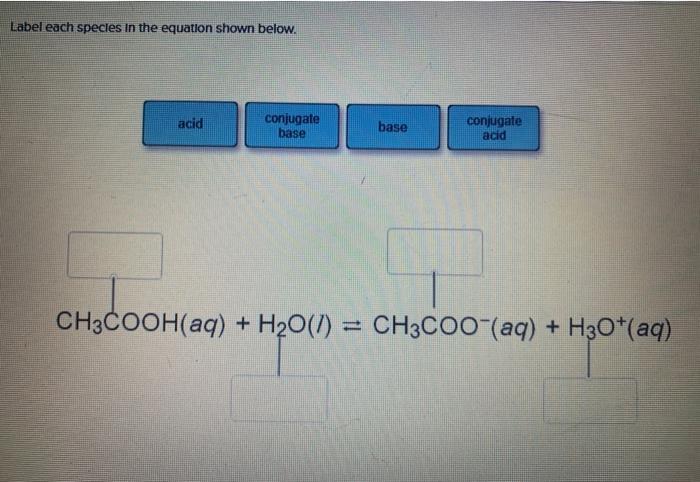
SOLVED: State whether aqueous solutions of the following salts produce acidic, basic or neutral solutions Justify your choice by referring to the specific properties of BOTH the cation and anion. You must
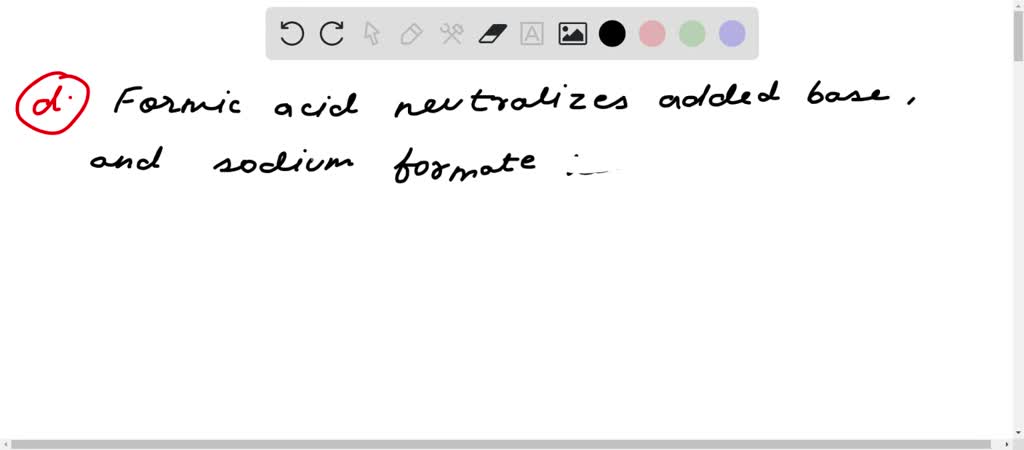
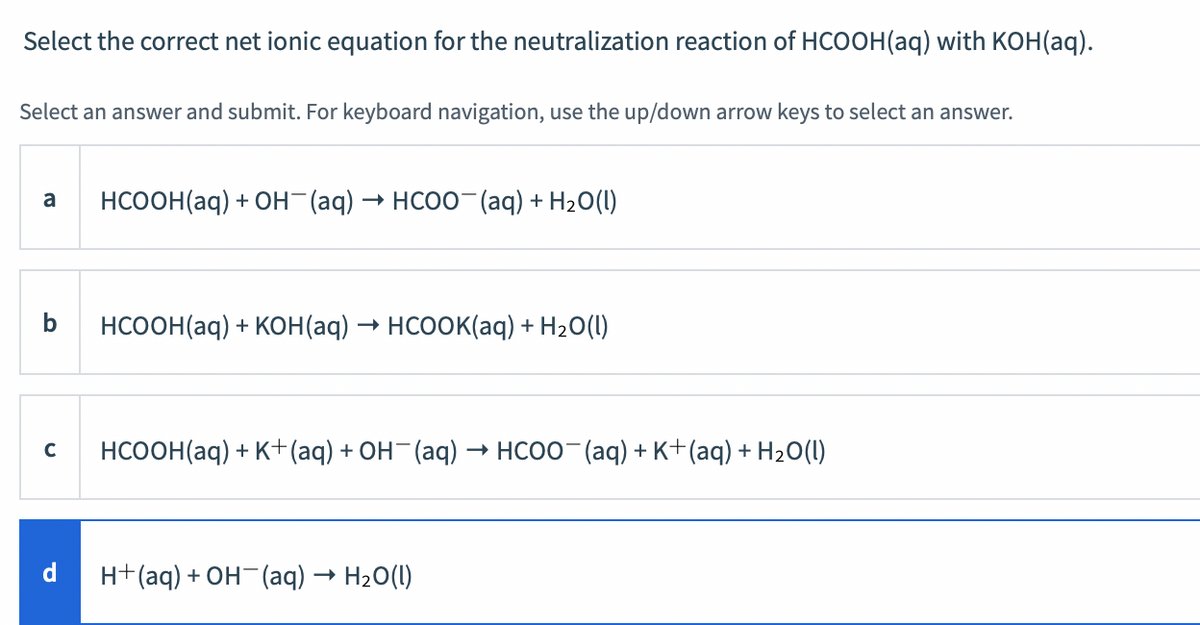
SOLVED: A buffer contains HCOOH (aq) and HCOOK (aq). Which statement correctly summarizes the action of this buffer? Both HCOOH (aq) and HCOOK (aq) neutralize added acid. Both HCOOH (aq) and HCOOK (

Interconversion between CO2 and HCOOH under Basic Conditions Catalyzed by PdAu Nanoparticles Supported by Amine-Functionalized Reduced Graphene Oxide as a Dual Catalyst | ACS Catalysis

Classify the following salt solution in acid, base and neutral. NaCl, KNO3 , FeCl3 , CuSO4 , CH3COONa, HCOOK, CH3COONH4 , CrCl3 , K2SO4 , Na3PO4 , NH4Cl
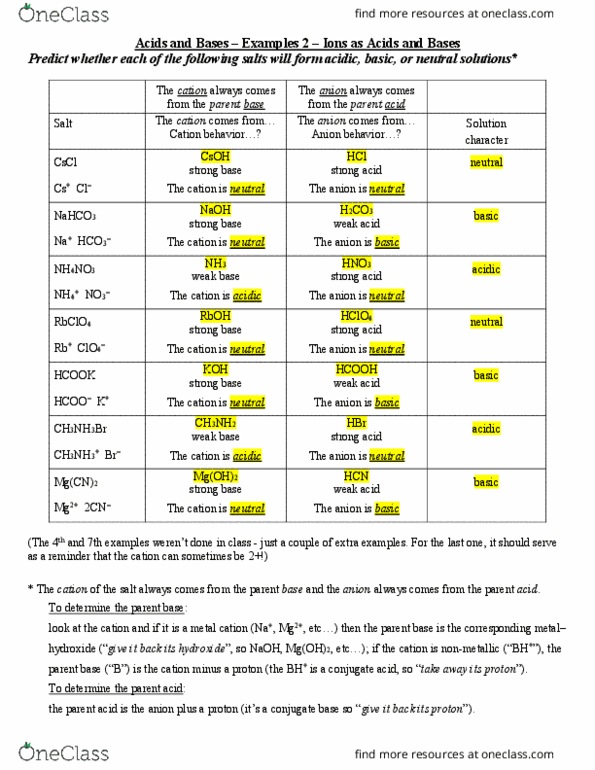
CHE 1302 Lecture Notes - Fall 2017, Lecture 12 - Equilibrium Constant, Hydrofluoric Acid, Rice Chart
Classify the following salt solution in acid, base and neutral. NaCl, KNO3 , FeCl3 , CuSO4 , CH3COONa, HCOOK, CH3COONH4 , CrCl3 , K2SO4 , Na3PO4 , NH4Cl