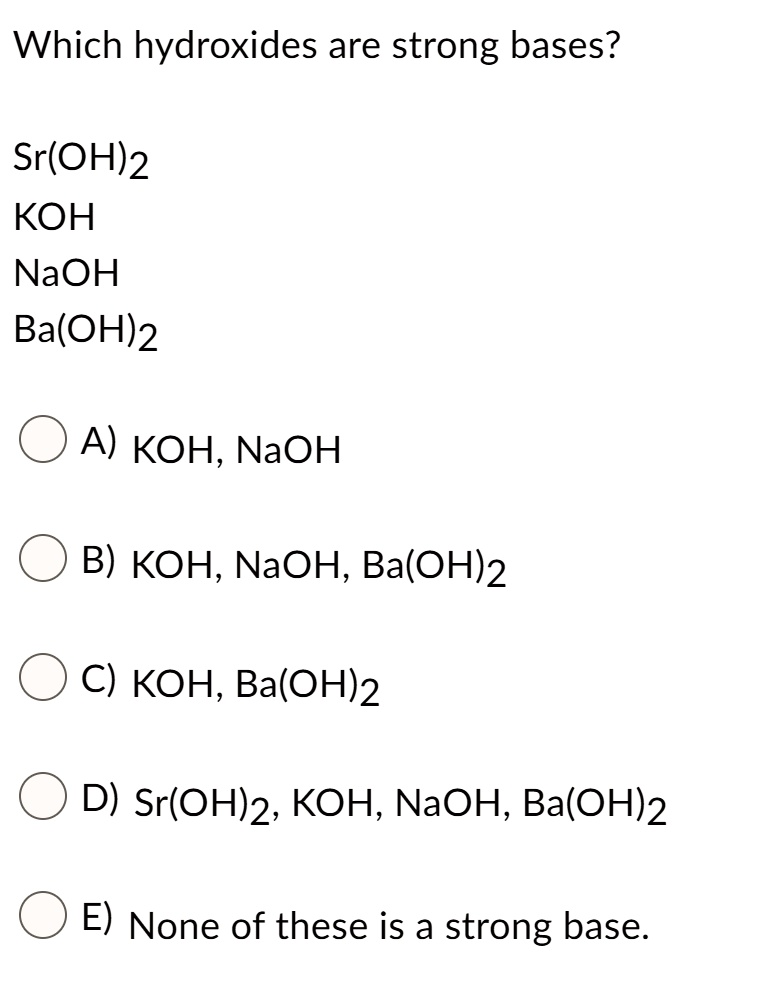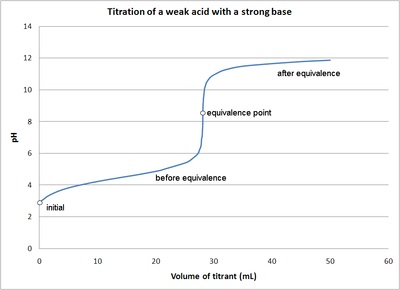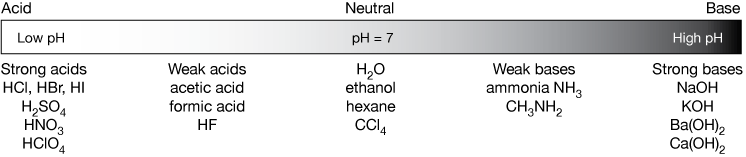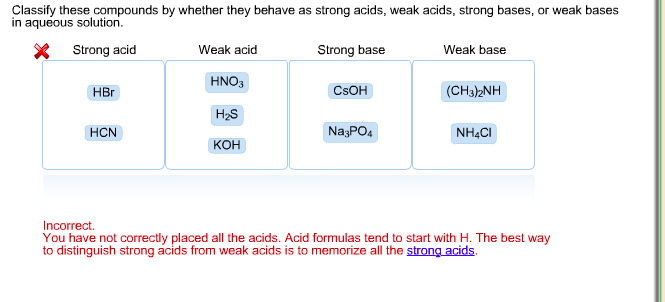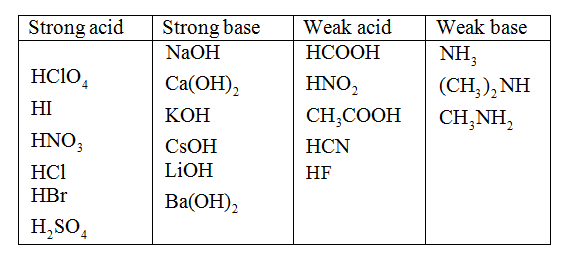
Classify each substance as a strong acid, strong base, weak acid, or weak base - Home Work Help - Learn CBSE Forum
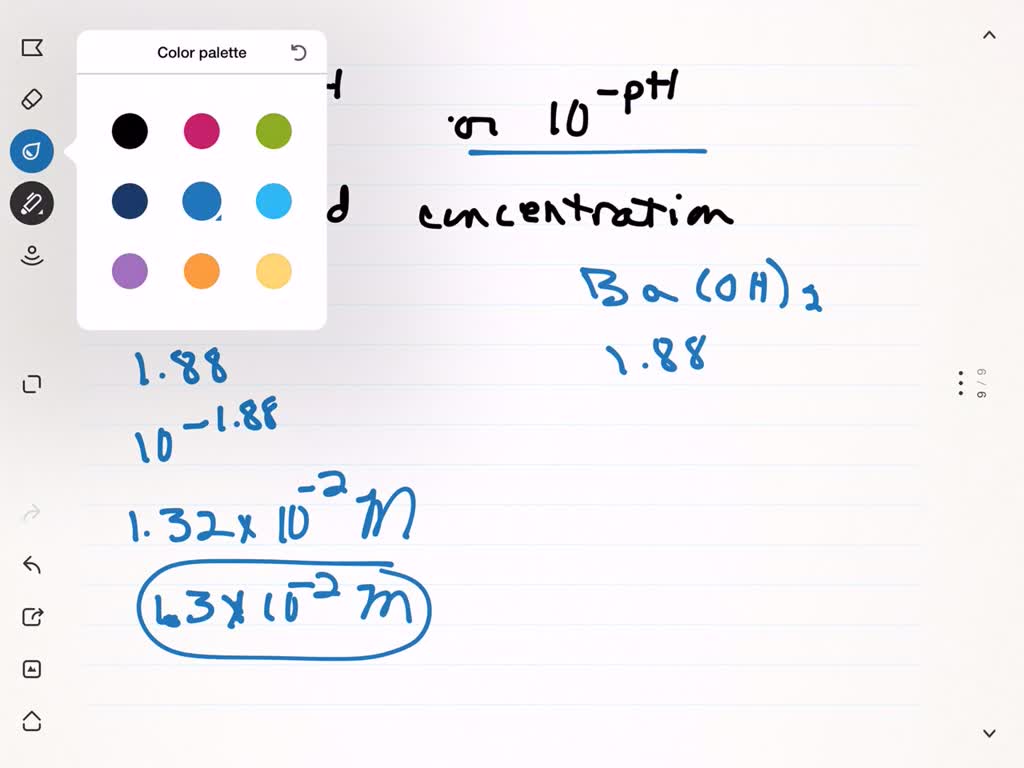
SOLVED:The pOH of a strong base solution is 1.88 at 25^∘ C. Calculate the concentration of the base (a) if the base is KOH and (b) if the base is Ba(OH)2.
Potassium hydroxide, caustic potash, lye molecule. KOH is strong caustic base and alkali, ionic compound. Structural chemical formula and molecule mod Stock Vector Image & Art - Alamy

OneClass: Strong acids Weak acids Strong bases Weak bases NaOH KOH HCOOH HNO3 HNO2 CH3NH2 C5H5N C2H C...
Potassium hydroxide, caustic potash, lye molecule. KOH is strong caustic base and alkali, ionic compound. Structural chemical formula and molecule mod Stock Vector Image & Art - Alamy