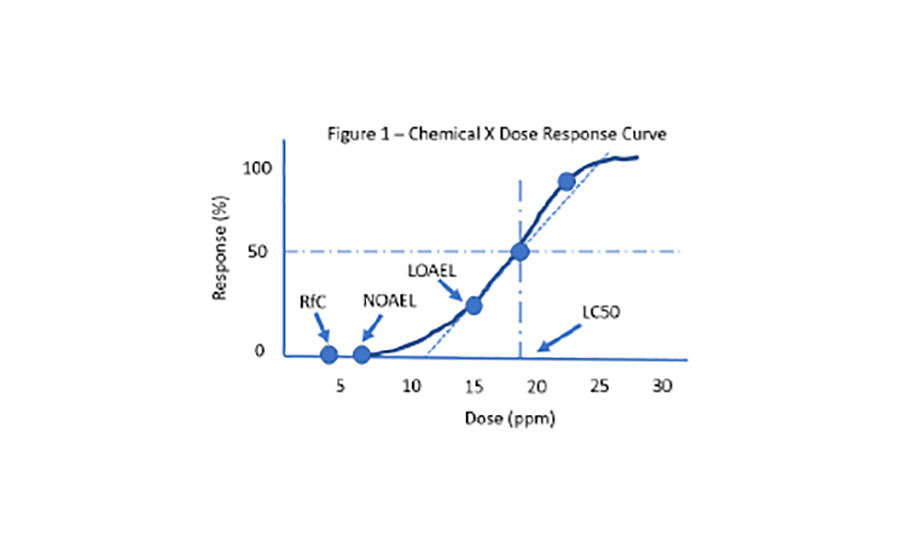
Calculation of the FIH dose from MABEL and NOAEL. The FIH dose from... | Download Scientific Diagram

Comparisons of NOAEL for humans for chronic inhalation and ingestion... | Download Scientific Diagram

Calculation of the FIH dose from MABEL and NOAEL. The FIH dose from... | Download Scientific Diagram

Do Similar Structures Have Similar No Observed Adverse Effect Level (NOAEL) Values? Exploring Chemoinformatics Approaches for Estimating NOAEL Bounds and Uncertainties | Chemical Research in Toxicology

Hormesis Shifts the No-Observed-Adverse-Effect Level (NOAEL) - Evgenios Agathokleous, Costas Saitanis, Athina Markouizou, 2021
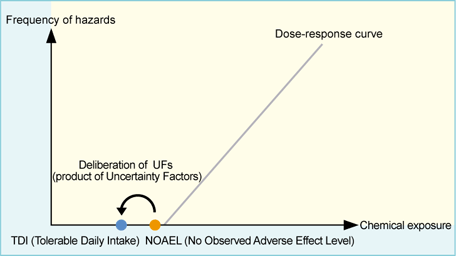
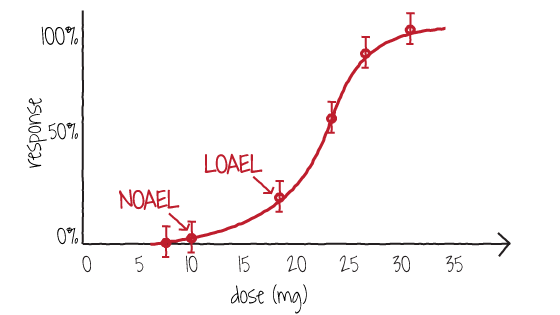
Risk Assessment on chemicals-For Better Understanding-4 | Chemical Management | National Institute of Technology and Evaluation (NITE)



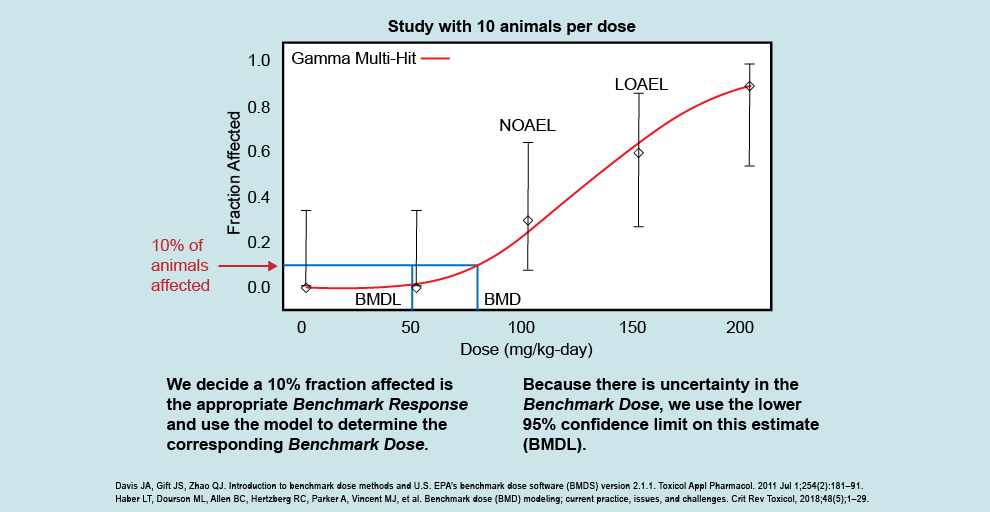



.png)